GERMANTOWN, MD / ACCESS Newswire / September 17, 2025 / uBriGene Biosciences, a leader in CGT CDMO and iPSC manufacturing technology development, today announced the launch of its 2nd-Generation iPSC Reprogramming Kit, powered by proprietary RNA-LNP technology. The kit is designed and proven to deliver best-in-class performance to reprogram peripheral blood mononuclear cells (PBMCs) to induced pluripotent stem cell (iPSC).
iPSC reprogramming diagram using uBriGene’s reprogramming mRNA-LNP cocktail mix.
Key Advantages of the 2nd-Generation RNA-LNP Reprogramming Kit:
-
RNA-LNP Based, Non-Viral Delivery – RNA-LNP delivery avoids genome integration risks, leaving no genetic footprint.
-
Optimized for PBMCs – The only non-viral kit proven effective for PBMC reprogramming, unlocking access to readily available patient samples.
-
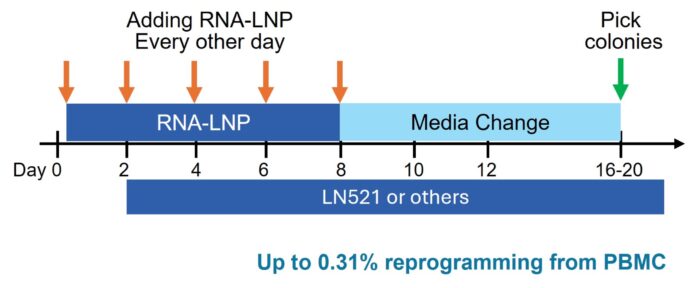
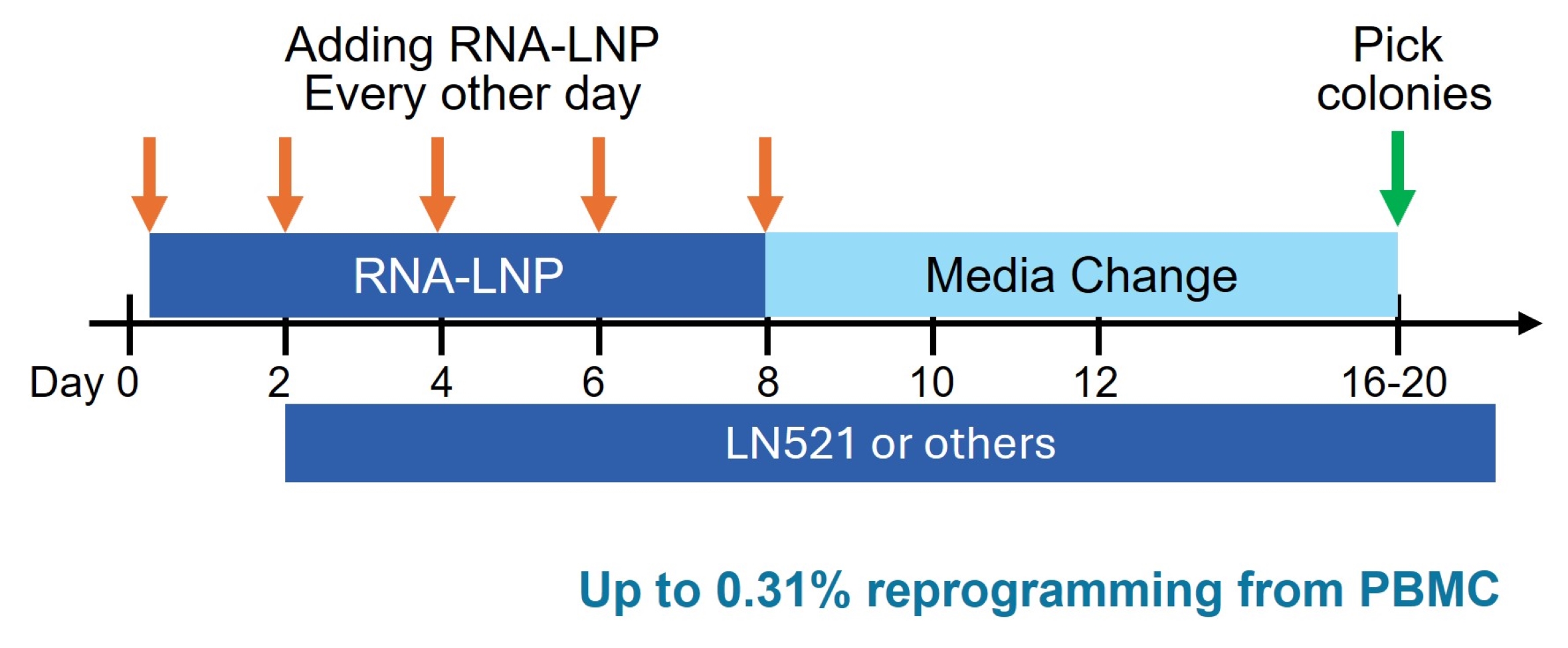
Exceptional Reprogramming Efficiency – Achieving up to 0.31% best-in-class efficiency in reprogramming PBMCs to iPSCs.
-
Autologous iPSC Therapies – This RNA-LNP reprogramming kit enables the generation of patient-specific iPSCs from just 10 mL of blood, eliminating the risk of immune rejection.
To learn more about this RNA-LNP breakthrough in PBMC reprogramming, visit iPSC Reprogramming Cocktail
“Until now, researchers seeking to generate iPSCs from PBMCs were limited to viral systems, such as Sendai virus, which pose regulatory and translational hurdles. uBriGene’s 2nd-Generation RNA-LNP Kit fills this unmet need by combining clinical-grade safety with high performance, accelerating the path from basic research to therapeutic applications,” commented Dr. Xiulian Sun, CTO and Founder of uBriGene Biosciences.
About uBriGene
Founded in 2015, uBriGene Biosciences is a leading Contract Development and Manufacturing Organization (CDMO) specializing in advanced therapeutic medicinal products (ATMPs). The company provides integrated CDMO and CRO solutions covering cell therapy products, viral vectors, RNA-related products, and induced pluripotent stem cell (iPSC) technologies. uBriGene’s iPSC portfolio includes RNA-LNP reprogramming kits, GMP-compliant iPSC banks, iPSC generation services, as well as iPSC banking to support research and clinical programs. Its GMP-validated Maryland facility provides one-stop services from process development through commercial manufacturing, driving global advancements in ATMPs and iPSC-based therapies.
Contact Information
Mingjuan Liu
Director of Marketing
contact@ubrigene.com
800 663 2528
SOURCE: UBRIGENE BIOSCIENCES INC
View the original press release on ACCESS Newswire